Affinity Biopharma recently announced the successful completion of its Series B2 financing round, raising over 400 million Chinese Yuan (CNY). This financing round was led by CS Capital, and jointly invested by China Growth Capital, Hangzhou Hongcheng Investment, SDIC Securities, Guosheng Capital, Hongfu Invest, Chengdu Anxin Guosheng Microchip Fund, Guoyuan Innovation Investment, and Hongyao Science and Technology. And CITIC Securities served as the financial advisor.
This funding round would boost the clinical development of the company’s globally innovative drugs, and accelerate the advancement of the company's technology platform and tumor microenvironment-activated therapies, aiming to address the substantial and urgent clinical needs in the field of tumor treatment.

About Affinity Biopharma:
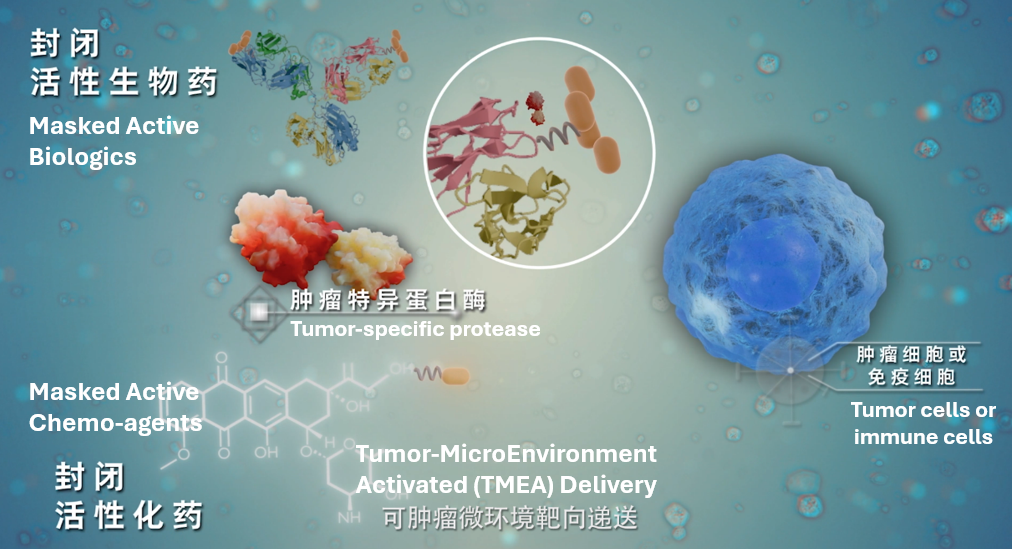
Affinity Biopharma is a clinical stage company focused on R&D, production and commercialization of the Tumor MicroEnvironment Activated (TMEA) platform, multispecific therapeutics and first-in-class anti-tumor treatment. With exclusive chemical conjugate linkers and validated proprietary platform, the company intends to unleash the active drugs specifically in the tumor local environment by tumor-specific proteases, and the platform could be applied to Small Molecule Drug Conjugates (SMDCs), antibodies, bispecific antibodies, cytokines and ADCs. The platform reduces “On target, Off tumor toxicity”, enhancing efficacy at targeted sites and dramatically improving the therapeutic index with global rights. Affinity Biopharma has developed a robust pipeline of over 20 innovative drug candidates targeting solid tumor indications.
Legubicin is a first-in-class, independently-developed Tumor-MicroEnvironment Activated (TMEA) small molecule drug conjugate (SMDC) with Doxorubicin (Dox) as payload, and its pivotal clinical trial will soon be unblinded. The interim analysis of the Phase III positive-control clinical trial of Legubicin in advanced soft tissue sarcoma (STS) revealed that, compared to the first-line Dox treatment, Legubicin has exhibited exceptional clinical performance and is expected to effectively improve the therapeutic window and safety profile. Notably, Legubicin fully addressed the cardiotoxicity challenges associated with anthracyclines and is anticipated to significantly prolong the progression-free survival (PFS) of patients. Multiple clinical studies of Legubicin were initiated simultaneously across a variety of cancer types.
Affinity Biopharma actively fosters collaborations and partnerships, including licensing out the China rights of a promising small-molecule TMEA drug and developing several collaborative innovative drugs through technology platform licensing. Leveraging its unique TMEA technology, the company aspires to develop world-leading, first-in-class, and best-in-class drugs and combination therapies, and is dedicated to improving the lives and outcomes for cancer patients worldwide.
Cheng Liu, M.D., Ph.D., Founder, CEO & Co-CMO of Affinity Biopharma:
“This financing round further demonstrates the strong recognition and optimism from investors for our tumor microenvironment-specific drug activation technology, intelligent drug development platform, and robust product pipelines. Our mission is to transform molecules with potentially effective yet highly toxic mechanisms of action to improve efficacy, reduce toxicities, and address the significant unmet medical needs in tumor treatment.
Additionally, we aim to unlock new opportunities for the expanded clinical applications of traditional drugs, the development of new molecular drugs, and the potential for innovative combination therapies. It is worth mentioning that the tumor-specific protease targets utilized in our platform are pan-tumor, highly expressed, and uniquely specific to tumor tissues, making them applicable to a wide range of solid tumor drug development initiatives.
In the next 10 to 20 years, we are committed to revolutionizing cancer treatment through our cutting-edge technology platform, striving to transform malignant tumors into chronic, manageable conditions that enable long-term survival.”
CS Capital Life Sciences team:
"Following the eras of chemotherapy, targeted therapy, and immunotherapy, conjugational drugs based on the design concept of 'targeting + potent killing' have ushered in a new chapter in tumor treatment. Affinity Biopharma has dedicated years to advancing legumain research, and its innovative conjugation platform significantly expands the therapeutic window of anti-tumor drugs, enhancing drug efficacy and upgrading traditional therapies into tumor site-activated and targeted versions.
The advantages of this technology have been initially validated through multiple clinical trials of Legubicin. CS Capital is committed to supporting the advancement of Affinity Biopharma’s existing pipeline and expanding its platform technology. We look forward to the company bringing in groundbreaking solutions in global drug conjugates, providing patients with safer and more effective treatment options, and driving the high-quality development of China’s innovative pharmaceutical industry."
Thanks to CS Capital, China Growth Capital, Hangzhou Hongcheng Investment, SDIC Securities, Guosheng Capital, Hongfu Invest, Chengdu Anxin Guosheng Microchip Fund, Guoyuan Innovation Investment, and Hongyao Science and Technology for their invaluable support in advancing Affinity Biopharma's innovative technology. Their contributions have been instrumental in driving clinical trials and improving outcomes for clinical patients.
-
2024/12/2 9:00:00Affinity Biopharma Announced the Completion of its Series B2 Financing Round Exceeding 400 Million Chinese Yuan (CNY) to Accelerate the Development of New Drugs
-
2023/7/5 10:35:54Affinity Biopharma Announced the Completion of B1 Round of Financing to Accelerate the Development of Innovative Product Pipelines
-
2021/10/29 10:22:58Affinity Participated in the 9th Healthcare and Life Sciences Leadership Summit held by China Renaissance


